PSA Testing Without Overtreating
Since 2008, healthcare providers have been instructed not to routinely use PSA testing on all men due to unnecessary biopsies and overtreatment of men with non-aggressive prostate cancer or benign conditions.
During that time:
- metastatic prostate cancer diagnoses have increased 5% annually
- prostate cancer deaths among American men have increased
- studies show a direct correlation between decreased use of PSA testing and increased late-stage diagnoses
Given this data — along with the development of molecular testing — we believe it is time for PCPs to reinstitute routine PSA screenings for men over age 50 at average risk and earlier screening for those with associated risk factors, including a family history of prostate cancer, ethnicity and genetic changes.
Effective Use of PSA Testing for Early Detection of PC
When to Start PSA Screening
In 2008, the US Preventive Services Task Force found that nearly 50% of men referred for biopsies had elevated PSA levels due to non-cancerous conditions or slow-growing prostate cancer, leading to its recommendation against routine PSA screenings. Since that time, prostate cancer biomarker tests have been developed that provide additional information on which patients should be referred for biopsy, reducing unnecessary biopsies by 40% or more.
We recommend use of these updated clinical guidelines to improve rates of early-stage prostate cancer diagnoses:

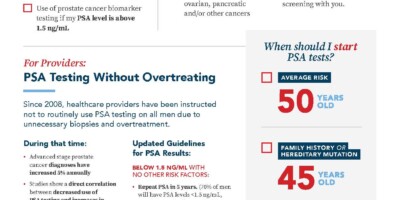
Average Risk
AGE 50

Family History
AGE 45

Black Male
AGE 40

Hereditary Mutation
AGE 45
Follow-Up for Abnormal PSA Results
We recommend using 1.5 ng/mL as the PSA cut-off. Over 70% of men will have PSA levels <1.5 ng/mL. If these patients have no other risk factors, this PSA results requires no further testing, PSA testing should be repeated in five years.
For PSA levels above 1.5 ng/mL, we recommend the following steps prior to biopsy:
- Digital rectal exam: PSA levels are correlated with prostate size, so checking for BPH will appropriately limit your pool of biopsy candidates
- Family history of prostate, breast, ovarian and/or colorectal cancer: Refer for genetic testing to check for known prostate cancer hereditary mutations
- Prostate cancer biomarker testing: If negative, repeat PSA in 1 year. If positive, refer to urologist
Post-PSA Biomarker Tests

Select MDx – Providers
Select mdx helps healthcare providers determine if a patient with an abnormal PSA is at higher or lower risk for prostate cancer and which patients can safely avoid biopsy.

4Kscore – Providers
The 4Kscore is a FDA approved test that offers the sensitivity and specificity needed to detect aggressive prostate cancer probability.

phi test – Providers
The Prostate Health Index (phi) test is an FDA-approved blood test that can help distinguish prostate cancer from benign prostatic conditions.

ExoDx – Providers
The ExoDx Prostate Test is a simple, non-DRE, urine-based, liquid biopsy test indicated for men 50 years of age and older.

ProstateNext – Providers
ProstateNext utilizes a blood or saliva to test for mutations in 14 genes that have been associated with hereditary prostate cancer.

PROSTest -Providers
Powered by a 27-gene panel and advanced machine learning, PROSTest offers a non-invasive, high-accuracy tool for diagnosis, biopsy triage, active surveillance, and post-treatment monitoring.
%s
FAQs for Providers About Post-PSA Biomarker Testing
The rate of advanced stage prostate cancer diagnoses has increased 5% annually since 2008 when routine PSA testing was stopped. Today we have more tools to detect PC in localized stages with 100% 5-year survival vs. 34% 5-year survival in advanced stage while reducing the risk of overtreatment.
Post-PSA biomarker tests provide an additional layer of data to inform the decision on whether to refer a patient with an abnormal PSA level for a urology consult and potential biopsy. Biomarkers should be used in conjunction with PSA results, health history, risk factors and medical exam (including DRE) to aid in early detection and intervention for improved patient outcomes.
Various health organizations recommend differing PSA cut-offs, leading to much of the confusion around PSA testing. Based on a study* reviewing the Henry Ford database for 22,000 men, including 30% African Americans, we found that men with a PSA below 1.5% had a .51% of developing a significant prostate cancer within 5 years. Based on this study, we recommend 1.5 ng/mL as the standard cut-off. 73% of patients will fall below that level, requiring no conversation and a re-check in 5 years. For the 17% above 1.5, we recommend an evaluation of DRE, biomarker testing and other risk factors to determine which patients should be referred for biopsy.
*Crawford ED et al. BJU Int 2011: 108(11): 1743-1747
Prior to ordering a PSA test, providers should discuss the patient’s prostate cancer risk factors — including average risk — as the basis for ordering the test. It’s important for providers to understand and note to their patients that 70% of men will require no further testing and that a higher-than-average PSA score does not result in an automatic referral for a biopsy. Like other standard screenings, such as lipid testing or blood pressure testing, PSA testing is an excellent first-line screening, but it should not be used in isolation to determine biopsy referrals.
Post-PSA biomarker testing is currently covered by Medicare and most major insurance plans. However, patients should always be reminded to check their insurance policies to verify coverage. Consult the table of PCMs provided on this page for general guidelines on insurance coverage or contact the test manufacturers, listed on the test ordering page, for additional details.
Provider Resources (Printable)
Below are resources providers can use in their practice to help guide prostate cancer screening decisions. We also have provided a patient flyer that will help you communicate important reasons for prostate cancer screening.
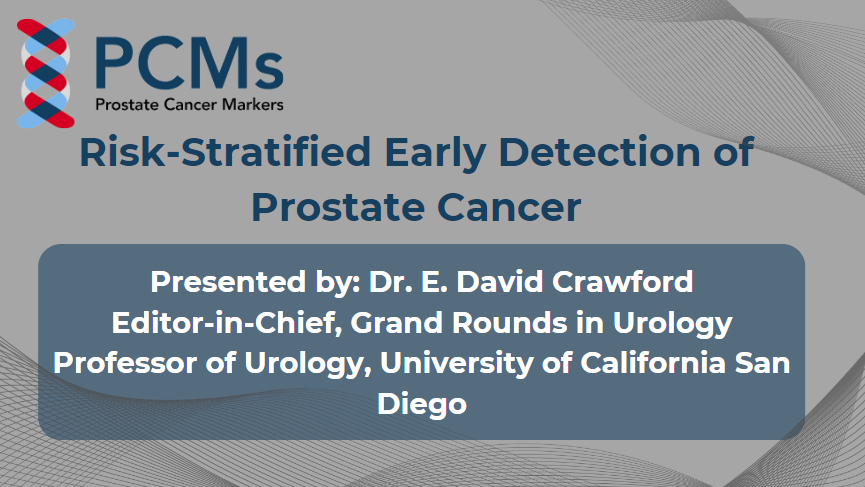
Phases of Prostate Cancer
Learn which prostate cancer biomarkers to use at various stages from screening to advanced cancer.
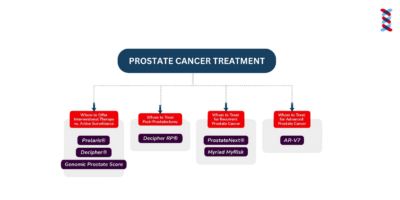
PCM Test Chart
The chart breaks down all PCM tests to display who they are targeted for and what results and decisions they provide.
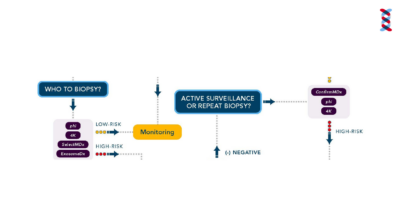
Prostate Cancer Algorithm
This algorithm helps providers understand the next steps from initial screening through treatment.
Patient's "Know Your Risk" Checklist
This flyer is meant for patients to print and use as a tool to assess their own risk, before visiting and talking through with their provider.
Related Journal Studies
An Approach Using PSA Levels of 1.5 ng/mL as the Cutoff for Prostate Cancer Screening in Primary Care
n this paper, we have presented an alternative approach in which screening is performed for men with at least a 10-year life expectancy. If the PSA is less than 1.5 ng/mL (approximately 70% of men who have a screening PSA), consider a 5-year rescreening interval. If the PSA is ≥ 1.5 ng/mL, or the PCP […]
96% Negative Predictive Value for High-grade Cancer
This study shows that, when compared to other risk factors, detection of DNA-methylation in histopathologically negative biopsies was the most significant and important predictor of high-grade cancer, resulting in a negative predictive value of 96%.
A Multi-Institutional Prospective Trial Confirms Noninvasive Blood Test Maintains Predictive Value in African American Men
The 4Kscore test accurately detects aggressive prostate cancer and reduces unnecessary biopsies yet, its performance in African American men has been unknown. We assessed test performance in a cohort of men with a large African American representation and confirmed that the 4Kscore test accurately predicts aggressive prostate cancer for biopsy decision making in African American […]
A multi-institutional prospective trial in the USA confirms that the 4Kscore accurately identifies men with high-grade prostate cancer
The 4Kscore combines measurement of four kallikreins in blood with clinical information as a measure of the probability of significant (Gleason ≥7) prostate cancer (PCa) before prostate biopsy. In this study, the 4Kscore showed excellent diagnostic performance in detecting significant PCa. It is a useful tool in selecting men who have significant disease and are […]
Beyond PSA: The Role of Prostate Health Index (phi)
Literature data showed that phi had good diagnostic performance to identify clinically significant (cs) PCa, suggesting that it could be a useful tool for personalized treatment decision-making. In this review, phi potentialities, limitations, and comparisons with other blood- and urinary-based tests were explored.
Commercialized Blood-, Urinary- and Tissue-Based Biomarker Tests for Prostate Cancer Diagnosis and Prognosis
In the diagnosis and prognosis of prostate cancer (PCa), the serum prostate-specific antigen test is widely used but is associated with low specificity. Therefore, blood-, urinary- and tissue-based biomarker tests have been developed, intended to be used in the diagnostic and prognostic setting of PCa. This review provides an overview of commercially available biomarker tests […]
Detection of High-grade Prostate Cancer Using a Urinary Molecular Biomarker-Based Risk Score
The risk score based on the mRNA liquid biopsy assay combined with traditional clinical risk factors identified men at risk of harboring high-grade PCa and resulted in a better patient risk stratification compared with current methods in clinical practice. Therefore, the risk score could reduce the number of unnecessary prostate biopsies.
Prostate Health Index and Prostate Health Index Density as Diagnostic Tools for Improved Prostate Cancer Detection
The objective of the present study was to evaluate the diagnostic potential of p2PSA, %p2PSA, phi, and phi density (PHID) as independent biomarkers and in combination with other demographic and clinical parameters, to predict overall and clinically significant PCa. The ability of p2PSA and its derivatives to discriminate HGPIN at biopsy was evaluated.
Prostate-specific antigen 1.5-4.0 ng/mL: a diagnostic challenge and danger zone
Both Caucasian and African American men with baseline PSA values between 1.5 and 4.0 ng/mL are at increased risk for future prostate cancer compared with those who have an initial PSA value below the 1.5 ng/mL threshold. Based on a growing body of literature and this analysis, it is recommended that a first PSA test threshold of 1.5 ng/mL and above, or somewhere between 1.5 and 4.0 ng/mL, represent the Early-Warning PSA Zone (EWP Zone).
The prostate health index selectively identifies clinically significant prostate cancer
We investigate whether phi improves specificity for detecting clinically significant prostate cancer and can help reduce prostate cancer over diagnosis. The test outperforms its individual components of total, free and [-2]proPSA for the identification of clinically significant prostate cancer. Phi may be useful as part of a multivariable approach to reduce prostate biopsies and over […]
%s
Related News Posts
The PSA Test Controversy, Doctor Vs Insurer on Settlements
In a lively recorded discussion, E. David Crawford MD explains the controversy around prostate-specific antigen (PSA) test screening. Listen to the full conversation on this episode of the Physician’s Weekly podcast.

Valley News: Are we getting it wrong on prostate cancer screening?
James Heffernan, an emeritus professor of English at Dartmouth College, speaks of his experience with prostate cancer and makes a case for early detection through prostate cancer biomarkers.

9News: Health officials urge people to get preventative screenings for prostate cancer
Dr. E. David Crawford, MD, on a featured story for CBS 9 News in Denver, Colorado regarding early PSA screening.

CBS 8 The Doctor is In: Men’s Prostate Health
Aditya Bagrodia, MD is a board-certified urologist who recommends using PCMarkers as a tool for both patients and providers to learn more about early detection for prostate cancer.

Who should get an at-home prostate-specific antigen test?
Blog post written by David Crawford, MD for Ash.com on at home diagnostic testing for prostate cancer.
American Cancer Society Releases Latest Cancer Statistics, Launches Initiative to Address Prostate Cancer Resurgence and Disparities
Prostate cancer, which is the second leading cause of cancer death for men in the U.S., increased by 3% per year from 2014 through 2019 after two decades of decline. Most concerning is that this increase was driven by the diagnosis of advanced disease. Since 2011, the diagnosis of advanced-stage (regional- or distant-stage) prostate cancer […]
LUGPA Pushes for Regular PSA Screening
Incidences of prostate cancer rose 3% annually from 2014 through 2019, translating to an additional 99,000 new cases and ending two decades of declines, the American Cancer Society reported earlier this year. About one in 8 men will be diagnosed with prostate cancer. The drop in prostate cancer screenings has played a role in the rise […]
Transgender Women at Risk for Prostate Cancer
A new study published in the Journal of the American Medical Association (JAMA) analyzed 22 years of data and found 14 prostate cancer cases per 10,000 transgender women. While a small study, it’s the first that looks at transgender women.
%s