The ExoDx test analyzes three biomarkers to help guide biopsy decision making in patients with elevated PSAs.
The ExoDx Prostate Test, EPI, is a urine-based, liquid biopsy test indicated for men 50 and older with a PSA of 2-10 ng/mL. This PSA range is ambivalent and considered a “grey zone”, with valid concern for referring men for biopsies who don’t have clinically significant prostate cancer. EPI measures exosomal RNA and provides a score that guides shared decision making over continued monitoring vs. prostate biopsy or mpMRI.
The test is included in the National Comprehensive Cancer Network (NCCN) guidelines. It has been clinically validated, with findings showing less than a 9% chance of having aggressive prostate cancer below the validated cut-point of 15.6.
ExoDx test benefits
- It is the only exosome-based test that provides unique, actionable intelligence to help you decide if biopsy is necessary; independent of PSA and other standard of care (SOC) features.
- The ExoDx Prostate Test does not require a digital rectal exam (DRE).
- The clinical validity of ExoDx is supported by multiple publications, including research published in Prostate Cancer and Prostatic Diseases and World Journal of Urology, representing approximately 5,000 patients from 40 academic and community urology clinics in the US.
ExoDx Prostate Test At A Glance
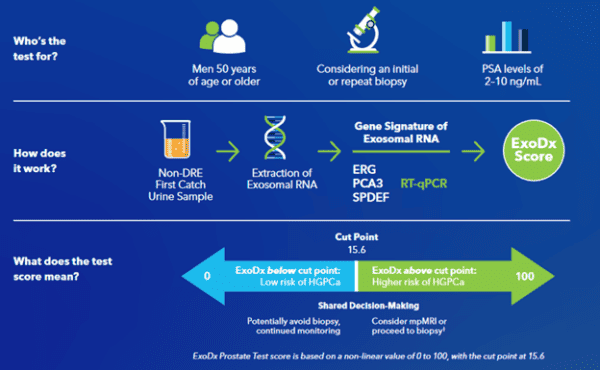
Results of the ExoDx Test will be sent to providers electronically via a HIPAA-compliant, secure online portal within 3-5 days of receiving the sample. ExoDx has representatives available 5 days a week to assist physicians with interpreting results.
What is unique about the ExoDX Test?
The ExoDx test is unique in that it is an exosome-based genomic test that offers an additional data point that is not obtained through nor influenced by the general work up (DRE, family history, PSA, or other standard of care features).
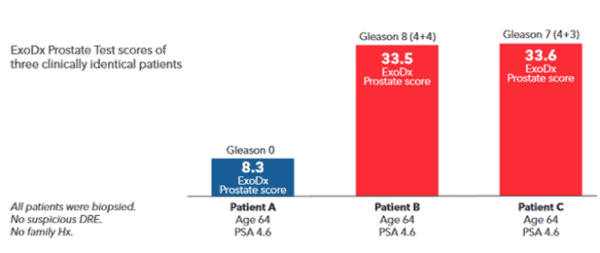
Patients who look clinically identical will have different ExoDx Prostate scores with varying levels of risk. This will result in unique, individualized patient conversations and, ultimately, more informed shared decision-making.
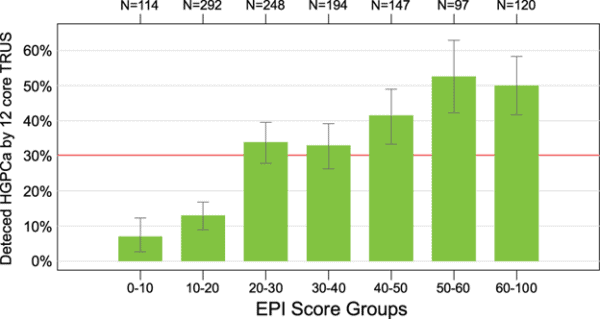
In addition to the above, according to the latest study published in Prostate Cancer and Prostatic Diseases in 2022, the validated cutoff of 15.6 would avoid 23% of all prostate biopsies and 30% of “unnecessary” (benign or Gleason 6/GG1) biopsies, with an NPV of 90% (as shown above in the chart)
How to Order the ExoDx test
Healthcare providers simply have to complete a HIPAA compliant electronic order form and can have the test kit sent to their office or to the patient’s home for self-collection. Visit https://www.exosomedx.com.
Learn More
Physician Resources
FAQs
Below are additional frequently asked questions about the ExosomeDx test.
The intended use population for the ExoDx Prostate Test is:
- Age 50 or over
- PSA of 2-10 ng/mL
- Considering an initial biopsy
The ExoDx test analyzes exosomal RNA from a non-invasive urine sample for three biomarkers known to be expressed in men with high-grade prostate cancer: ERG, PCA3 and SPDEF. You should consult your physician or healthcare provider when reviewing the results of your ExoDx test.
The ExoDx Score is returned as above or below 15.6, which is the test’s cutoff for high grade prostate cancer.
- If below 15.6, there is a decreased likelihood of greater than or equal to Gleason 7 prostate cancer on biopsy.
- If above 15.6, there is an increased likelihood of greater than or equal to Gleason 7 prostate cancer on biopsy.
See a sample ExoDx test result report and learn more about how to interpret these scores.
No. One of the key benefits of the ExoDx Prostate Test is that it does not require you to have a digital rectal exam (DRE) prior to providing a urine sample for the test. In fact, if you do have a DRE, then you must wait 24 hours before you are able to provide a urine sample for the ExoDx Prostate Test.
Studies Involving ExoDx
Pre-diagnosis urine exosomal RNA (ExoDx EPI score) is associated with post-prostatectomy pathology outcome
ExoDx Prostate IntelliScore (EPI) is a non-invasive urine exosome RNA-based test for risk assessment of high-grade prostate cancer. We evaluated the association of pre-biopsy test results with post-radical prostatectomy (RP) outcomes to understand the potential utility of EPI to inform invasive treatment vs active surveillance (AS) decisions.
Predicting high-grade prostate cancer at initial biopsy: clinical performance of the ExoDx (EPI) Prostate Intelliscore test in three independent prospective studies
The ExoDx Prostate (IntelliScore) (EPI) test stratifies patients for risk of high-grade prostate cancer (HGPC; ≥ Grade Group 2 [GG] PC) in men ≥ 50 years with equivocal prostate-specific antigen (PSA) (2–10 ng/mL). Here, we present a pooled meta-analysis from three independent prospective-validation studies in men presenting for initial biopsy decision.
%s
