The AR-V7 is a liquid biopsy blood test that identifies the presence of AR-V7, an important emerging biomarker in castrate resistant prostate cancer (CRPC). For healthcare providers with patients with mCRPC who have failed at least one line of an AR-signaling inhibitor (ARSi) treatment, the AR-V7 test can provide clarity on whether or not to pursue additional ARSis or consider chemotherapy. 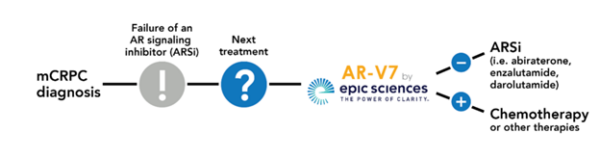
AR-V7 Test Benefits
- Only requires a liquid biopsy (blood) sample
- Accurately identifies men who would not respond favorably to AR-signaling inhibitor therapy
- Is associated with improved outcomes with taxane treatment for men who are not receptive to AR-signaling inhibitors
- Provides a positive or negative test result, making it easy to interpret
AR-V7 Physician Video
Learn more about the AR-V7 test.
For Healthcare Providers: How to Order the AR-V7 Test
Healthcare providers can place orders for the AR-V7 test online at Epic Sciences’ website here.
Below are additional frequently asked questions about the AR-V7 test
Patients who have mCRPC (metastatic castration-resistant prostate cancer) may consider the AR-V7 test to determine if they will NOT respond to androgen receptor (AR) targeted therapies, such as abiraterone and enzalutamide, and should consider chemotherapy instead.
While patients should always discuss the results of their prostate cancer test with their healthcare provider, in general, a negative test result for the AR-V7 test means they are NOT resistant to androgen-receptor targeted therapy and could consider the treatment. If their test is positive, it means they are resistant to androgen-receptor targeted therapy and may not benefit from this targeted hormonal therapy. If that is the case, the patient and their healthcare provider may consider other treatments such as taxane chemotherapy. See a sample ARV-7 test and learn more about how to interpret the results.
Most major insurance companies cover the AR-V7 test. Epic Sciences has a Patient Financial Assistance Program to help patients with out-of-pocket costs. Please note that patients with Medicaid or Medicare are not eligible for the financial assistance program
Through a blood sample, Epic Sciences’ technology analyzes almost all of the circulating tumor cells and can accurately predict their response or resistance to androgen-receptor signaling inhibitor (ARSi) therapies such as abiraterone, enzalutamide, or apalutamide. These insights can help inform a healthcare provider’s recommended treatment.
Learn more
Explore more information about the AR-V7 test.
Association of AR-V7 on Circulating Tumor Cells as a Treatment-Specific Biomarker With Outcomes and Survival in Castration-Resistant Prostate Cancer
This study of 161 men with metastatic castration-resistant prostate cancer (mCRPC) validates circulating tumor cells (CTC) nuclear expression of AR-V7 protein as a treatment-specific biomarker that is associated with better survival rates for patients on taxane therapy versus androgen receptor signaling (ARS)-directed therapy.
Assessment of the Validity of Nuclear-Localized Androgen Receptor Splice Variant 7 in Circulating Tumor Cells as a Predictive Biomarker for Castration-Resistant Prostate Cancer
This study of 142 men with metastatic castration-resistant prostate cancer (mCRPC) found that nuclear-localized AR-V7 protein in circulating tumor cells can be an indication of whether or not these patients have better survival rates with taxane chemotherapy compared to androgen receptor signaling (ARS)-directed therapy.
%s