The 4Kscore test combines results from 4 biomarkers with clinical results to provide specificity predict the likelihood of finding aggressive prostate cancer in a biopsy.
The 4Kscore is a blood test that incorporates clinical results with measurements of four prostate-specific biomarkers: Total PSA, Free PSA, Intact PSA, and human kallikrein 2 (hk2). Total PSA and Free PSA are important markers for prostate cancer but have low specificity detecting the risk of aggressive prostate cancer. Intact PSA and human kallikrein 2 (hk2) are present in much lower concentrations than total PSA and Free PSA, and changes in these biomarkers are associated with aggressive types of prostate cancer.
4Kscore® Test benefits
- The 4Kscore is a FDA approved test offers the sensitivity and specificity needed to detect aggressive prostate cancer probability.
- The 4Kscore Test is clinically validated in two U.S.–based, prospective studies, including a 2017 study where the majority of subjects were African American.
- When probability score of 7.5% is used for assessment, no Gleason ≥8 was missed.
- The study shows that a significant number of patients could have avoided unnecessary biopsies.
- The 4Kscore Test can probability-stratify men for prostate cancer metastasis and mortality for up to 20 years, according to research published in the Journal of Urology.
- Multiple peer-reviewed publications, with studies involving more than 20,000 men, demonstrate the clinical effectiveness of the 4Kscore Test.
- 4Kscore Test is included in current U.S., Canadian, and European prostate cancer early-detection guidelines.
For Healthcare Providers: How to Order the 4Kscore Test
Healthcare providers can visit www.4kscore.com or call 1-833-457-2673
Below are additional frequently asked questions about the 4Kscore Test.
The 4Kscore test is covered by most leading healthcare insurance plans. Your provider will bill your insurance company, and you will only be responsible for applicable copayment, co-insurance, and/or deductible amounts. For non-covered services, BioReference Laboratories, Inc. and their GenPath division also have self-pay and payment plan options available.
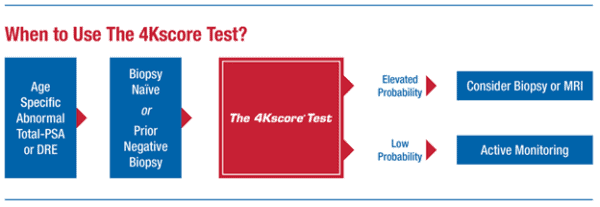
The 4Kscore test is appropriate for the following men:
- Men 45-54 years old and total PSA ≥2 ng/mL and/or abnormal DRE
- Men 55-75 years old and total PSA ≥3 ng/mL and/or abnormal DRE
- Men ≥76 years old and total PSA ≥4 ng/mL and/or abnormal DRE
The 4Kscore test is not indicated in men with:
- Previous diagnosis of prostate cancer
- DRE performed in the previous 96 hours before phlebotomy
- 5-alpha reductase medication within previous 6 months
- Prostate procedure within the last 6 months
Numerous studies have confirmed the effectiveness of the 4Kscore test in predicting aggressive prostate cancer on a biopsy, including:
- -Parekh et al. A multi-institutional prospective trial in the USA confirms that the 4Kscore® accurately identifies men with high-grade prostate cancer. Eur Urol. 2015;68(3):464-470.
- Punnen et al. A multi-institutional prospective trial confirms noninvasive blood test maintains predictive value in African American men. J Urol. 2018;199(6);1459-1463.
Learn More
Explore more information about the 4Kscore test.
Studies
A Multi-Institutional Prospective Trial Confirms Noninvasive Blood Test Maintains Predictive Value in African American Men
The 4Kscore test accurately detects aggressive prostate cancer and reduces unnecessary biopsies yet, its performance in African American men has been unknown. We assessed test performance in a cohort of men with a large African American representation and confirmed that the 4Kscore test accurately predicts aggressive prostate cancer for biopsy decision making in African American […]
A multi-institutional prospective trial in the USA confirms that the 4Kscore accurately identifies men with high-grade prostate cancer
The 4Kscore combines measurement of four kallikreins in blood with clinical information as a measure of the probability of significant (Gleason ≥7) prostate cancer (PCa) before prostate biopsy. In this study, the 4Kscore showed excellent diagnostic performance in detecting significant PCa. It is a useful tool in selecting men who have significant disease and are […]
%s
