A Call for Reinstating Standard Prostate Cancer Screenings
Increasing metastatic prostate cancer rates linked to reduced PSA screenings
While routine PSA testing has significantly declined since 2008, the incidence of metastatic prostate cancer has increased for men 45 and older across all races, ethnicities, and geographic locations in the US. (1) Multiple studies, including the American Cancer Society’s 2023 Cancer Statistics report, show a strong correlation between the decrease in PSA screening and an increase in metastatic prostate cancer.(2)
Given this data — along with the development of molecular testing — we believe it is time for PCPs to reinstitute routine PSA screenings for men over age 50 at average risk and earlier screening for those with associated risk factors, including a family history of prostate cancer, ethnicity and genetic changes. We also argue that PSA screenings should be handled in the same manner as routine lipid or blood pressure tests. In other words, informed consent is not necessary because the vast majority of men, in fact 73% of men, will require no discussion because their PSA will be below 1.5 ng/mL and will have less than 0.51% chance of developing prostate cancer in the following 5 years.(3)
Two Factors Support Reinstatement of Routine PSA Testing
In 2012, the US Preventive Services Task Force (USPSTF) gave PSA testing a Grade D recommendation, saying the harm of the screening outweighed potential benefits. In 2018, the task force slightly upgraded the recommendation to a C, saying that men ages 55-69 years old should make an individual, informed decision about PSA testing with their provider.
So why are we advocating a return to routine PSA testing? Two factors are driving the call:
- The rate of advanced prostate cancer is increasing up to 5% annually and the number of men with distant-stage cancer has doubled since 2011, according to the American Cancer Society. Although prostate cancer has a 5-year survival rate of 100% when found early, that number drops to 34% in cases where prostate cancer spreads to a distant site, according to the National Cancer Institute Cancer Stat Facts: Prostate Cancer. In short, men are dying needlessly from prostate cancer, already the second leading cause of cancer deaths in men.
- New, non-invasive biomarker tests can provide additional insights about cancer risk BEFORE and after a biopsy. In fact, a study published in Prostate Cancer and Prostatic Diseases found the prostate cancer marker (PCM) test known as the Prostate health index (phi), reduced unnecessary biopsies by nearly half. And the phi test is just one of many PCMs available today.
PSA Misperceptions Put More Men At Risk
More than a dozen provider and patient organizations have issued differing recommendations about PSA testing, primarily due to outdated information and a misperception that the PSA isn’t a valid screening tool since it does not give a definitive answer about whether prostate cancer is present.
The PSA alone doesn’t give you, the PCP, clear direction on next steps. Yet for years, the PSA, often along with an abnormal digital rectal exam (DRE), led almost immediately to a prostate biopsy. Many men, who would likely have never developed malignant prostate cancer, were left with long-term consequences of a biopsy including bleeding, discomfort, impotence, incontinence, or other negative side effects. The USPSTF found that up to 50% of men with elevated PSAs were overtreated, which led to its recommendations.
In the decade since these recommendations were issued, we have seen the rate of PSA testing decline while the rate of metastatic prostate cancer increase. A study published in 2022 in JAMA “demonstrated decreasing (prostate cancer-specific mortality) rates that flattened or increased after the 2012 USPSTF Grade D recommendation, suggesting that decreased PSA screening may be a factor associated with this change. This change was seen across ages, races and ethnicities, urbanization categories, and US Census regions.”(1)
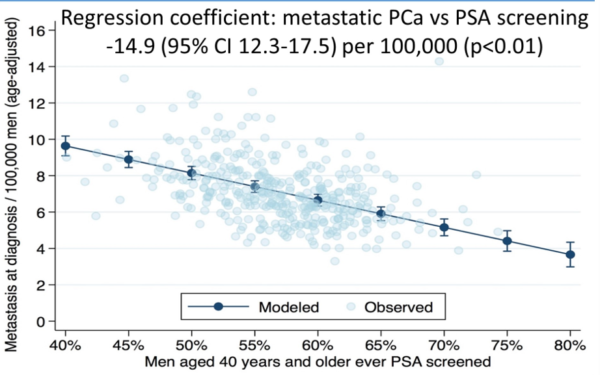
A presentation made at the 2021 ASCO Genitourinary Cancers Symposium found: “A random-effects linear regression model demonstrated that longitudinal reductions across states in PSA screening were associated with increased metastatic prostate cancer.” (2) See figure below.
How Prostate Cancer Biomarker Testing Changes the Playing Field
The second factor that supports reinstatement of routine PSA testing is the development of prostate cancer biomarker tests, also called prostate cancer markers (PCMs). These tests look for molecules in the blood, urine, or tissue that can signify normal or abnormal activity. PCMs provide valuable insights, such as:
- differentiate between cancer and benign conditions such as benign prostatic hyperplasia (BPH)
- indicate if aggressive prostate cancer might be found on a biopsy
- predict if prostate cancer may be receptive to certain treatments.
For you as a PCP, PCMs give you and your patient additional information to make more informed decisions. PCMs allow you to feel more confident about recommending a prostate biopsy, MRI, or other next steps.
Prostate biomarkers are being developed regularly. Some of the current biomarkers being measured are:
- Kallikreins
- Biomarkers associated with high-grade prostate cancer including ERG, PCA3 and SPDEF
- HOXC6 and DLX1
- AR-V7
- BRCA1 and BRCA2
- RNA biomarkers associated with metastatic progression
Biomarker Testing for Patients With Elevated PSA Levels
Before recommending a biopsy, consider these PCMs before referring for a prostate biopsy:
- Select mdx: This urine test looks for two biomarkers that could indicate if a patient may be at increased risk of cancer and should have a biopsy.
- 4K: This blood test can help differentiate if a high PSA is due to a benign condition or cancer.
- Prostate health index (phi): This blood test can better differentiate between benign conditions and prostate cancer and is especially helpful for men with a PSA between 4-10 ng/mL.
- ExoDx: This urine-based tests looks for biomarkers associated with high-grade (aggressive) prostate cancer.
If your patient has a family history of prostate cancer, breast cancer, pancreatic cancer, ovarian cancer or colorectal cancer, germline testing should be considered to determine the risk of hereditary cancer. Current germline tests include:
- Prompt PGS
- Myriad myRisk
- ProstateNext
- Ambry Score
